![Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube](https://i.ytimg.com/vi/R5RDFu1oYUU/maxresdefault.jpg)
Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube
Explain [Co(NH_3)_6]^{3+} is an inner orbital complex whereas [Ni(NH_3)_6]^{2+} is an outer orbital complex.
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:598/0*gC7TqoG4Szy6QwnB.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
86. The formation constant of Ni(NH3)6 is 6 ×10^8 at 25^° C. If 50 ml of 2.0 M NH_3, is added to 50 ml of 0.20M solution of Ni^2+, the concentration of
![SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light. SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.
![C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/3c02ac26-f23b-4dda-96f6-23fb3b82533e.jpg)
C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B

The formation constant of `Ni(NH_3)_6^(2+)` is `6xx10^8` at `25^@C`.If 50 ml of 2.0 M `NH_3` is - YouTube
Coordination compound: Explain [Co(NH3)6]3+ is an inner orbital complex while [Ni(NH3)6]2+ is an outer orbital complex .

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 Palladium Plutonium Darmstadtium balanced equations GCE AS A2 IB A level inorganic chemistry revision
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1280/0*8wwL8Ru43LyLIIL8.png)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.](https://cyberleninka.org/viewer_images/173973/f/1.png)
Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.
![Explain the hybridisation, magnetic property and geometry { left[ Ni{ left( CN right) }_{ 4 } right] }^{ 2- } and { left[ Ni{ left( { NH }_{ 3 } right) }_{ 4 } right] }^{ 2+ } using VB theory. Explain the hybridisation, magnetic property and geometry { left[ Ni{ left( CN right) }_{ 4 } right] }^{ 2- } and { left[ Ni{ left( { NH }_{ 3 } right) }_{ 4 } right] }^{ 2+ } using VB theory.](https://search-static.byjusweb.com/question-images/toppr_ext/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
![why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE](https://preview.redd.it/why-cant-ni-nh3-6-2-have-dsp3-hybridization-then-v0-slwfvbv55ria1.png?width=640&crop=smart&auto=webp&s=f4e658df50fc25da185b4ccd9590bfb69fc4c902)
![Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=676374042957917)

2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)
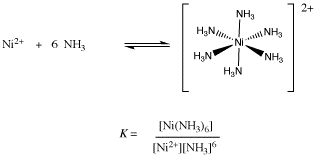


![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)
![Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
![Ni(NH3)6]+3 hybridisation structure - YouTube Ni(NH3)6]+3 hybridisation structure - YouTube](https://i.ytimg.com/vi/iZYGIu-3Fls/maxresdefault.jpg)
